 The Byzantine array of interconnected networks that modulate normal cellular function are all crucially dependent on biomolecular recognition. Non-covalent complexes among and between proteins, nucleic acids, and carbohydrates influence virtually all cellular processes. An in-depth understanding of the forces that govern these recognition events is thus a logical stepping stone to the precise observation and perturbation of biology.
The Byzantine array of interconnected networks that modulate normal cellular function are all crucially dependent on biomolecular recognition. Non-covalent complexes among and between proteins, nucleic acids, and carbohydrates influence virtually all cellular processes. An in-depth understanding of the forces that govern these recognition events is thus a logical stepping stone to the precise observation and perturbation of biology.
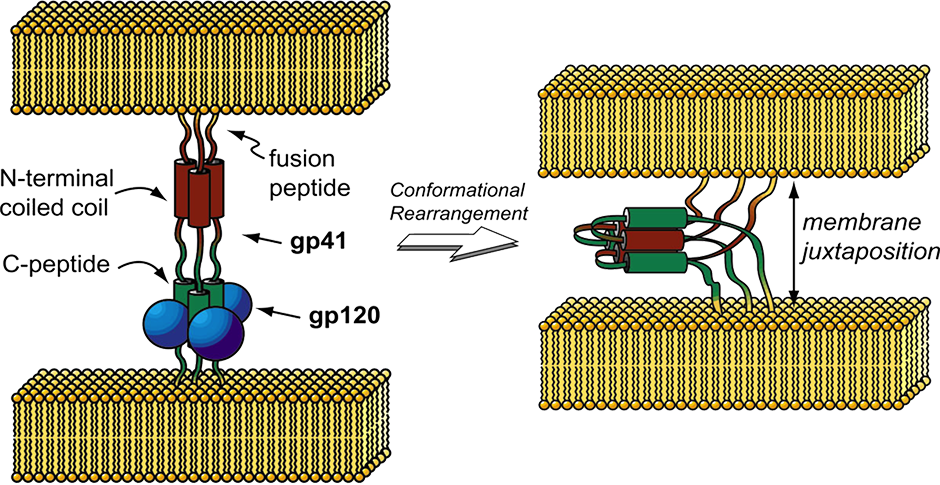
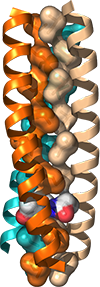
We are interested in the molecular details that underlie non-covalent peptide and protein self-assembly, particularly as manifested in the ubiquitous alpha-helical coiled coil protein fold. We have examined a variety of structure-function relationships between side chain structure and assembly behavior. More recently we have been interested in recognition events at the surface of coiled coils, especially in the context of Class I fusion proteins that mediate infection in a broad range of enveloped viruses. This group includes many pathogens of significant menace to human health (e. g., HIV, Ebola), and all operate by a common trimer-of-hairpins mechanism the features an obligate recognition between a peptide ligand and a pre-formed trimeric coiled coil. We are currently exploring methods for high-throughput discovery of new viral protein ligands and leveraging known structures for the design of new bio-orthogonal recognition motifs.